SECURITY SEALS AND LABELS FOR PHARMACEUTICAL CONTAINERS
Seals for pharmaceutical containers
The counterfeiting of pharmaceutical products is an enormous risk for consumers’ health and a major economic loss for the pharmaceutical industry. The safety of pharmaceutical products is a necessity for the final consumer and it requires an utmost control of the flow of products, from the manufacturing to the distribution.
In this context, many countries have decided to adopt new regulations with the aim to introduce safety elements for the packaging of medicinal products for human use. This is a significant change that will ensure the authenticity of medicines and safety for patients (and also for the pharmaceutical industry) and will strengthen the security of the supply chain.
The integrity of the tamper prevention system ensures that the packaging has not been opened or altered after its release from the production facilities, thus ensuring the integrity of the contained drug.
In 2011, the European Union issued a Specification concerning the Falsified Medicines (DIRECTIVE 2011-62-EU to replace the previous 2001-82-EU) which indicated the key points of anti-counterfeiting that can be simplified in the concepts related to “Safety Features”such as: Authenticity, Identification, Tampering.
The European Directive on Falsified Medicines (FMD) makes the serialization and tamper evident processes mandatory for every drug that will enter the European single market from February 2019. For this reason, even some non-EU member countries such as Switzerland, Norway and Iceland have decided to adapt to the FMD.
In general, every pharmaceutical manufacturer will be required to comply with the regulations of the markets in which he wants to sell his products. For this reason, pharmaceutical companies that export their products abroad are subject to these regulations even if the serialization is not yet regulated by their local legislation.
Seals for pharmaceutical containers
The counterfeiting of pharmaceutical products is an enormous risk for consumers’ health and a major economic loss for the pharmaceutical industry. The safety of pharmaceutical products is a necessity for the final consumer and it requires an utmost control of the flow of products, from the manufacturing to the distribution.
In this context, many countries have decided to adopt new regulations with the aim to introduce safety elements for the packaging of medicinal products for human use. This is a significant change that will ensure the authenticity of medicines and safety for patients (and also for the pharmaceutical industry) and will strengthen the security of the supply chain.
The integrity of the tamper prevention system ensures that the packaging has not been opened or altered after its release from the production facilities, thus ensuring the integrity of the contained drug.
In 2011, the European Union issued a Specification concerning the Falsified Medicines (DIRECTIVE 2011-62-EU to replace the previous 2001-82-EU) which indicated the key points of anti-counterfeiting that can be simplified in the concepts related to “Safety Features”such as: Authenticity, Identification, Tampering.
The European Directive on Falsified Medicines (FMD) makes the serialization and tamper evident processes mandatory for every drug that will enter the European single market from February 2019. For this reason, even some non-EU member countries such as Switzerland, Norway and Iceland have decided to adapt to the FMD.
In general, every pharmaceutical manufacturer will be required to comply with the regulations of the markets in which he wants to sell his products. For this reason, pharmaceutical companies that export their products abroad are subject to these regulations even if the serialization is not yet regulated by their local legislation.
Tamper Evident solutions: Arca's offer
In compliance with Directive 2011-62-CE, Arca Etichette’s offer is structured with a range of solutions for pharmaceutical serialization and for the anti-tamper of pharmaceutical boxes and containers.
For this requirement, Arca can offer two highly effective tamper-evident solutions: Wipe-Out & Void seals/labels.
Even larger surface areas, such as boxes containing products or other bulky packaging, can be protected using Wipe-Out and Void type tamper-proof security tapes.
The wide range of materials for security label production and printing allows a safe use both on flat surfaces and on corner applications.
Our tamper-evident labels can also be combined with special security inks, that can function as anti-counterfeiting and security elements themselves.
Wipe-out seals
A real innovation, patented by Arca Etichette and designed to effectively cope with the problem of tampering, these labels can seal any type of box and container, making evident all attempts at illegal break-ins, thus discouraging product and content theft and sophistication.
The particular combination of materials and the specific types of adhesives used make it virtually impossible to detach the label from the container without breaking it into pieces, avoiding its repositioning after the break-in.
Wipe-Out labels comprise two layers:
- A lower layer made of a fragile destructible material
- An upper layer made of a transparent plastic material
If any attempt is made to remove it, the label will effectively be destroyed by three joint factors:
- the plastic layer tends to separate from the lower paper layer, stripping away the print;
- the lower layer tends to self-destruct, due to its inherent fragility;
- the destruction of the lower layer is ensured by the special pattern of pre-cuts over the entire label, extending to its perimeter.
For applications on medicine containers, Arca Etichette offers a range of materials and adhesives specifically designed for the pharmaceutical industry.
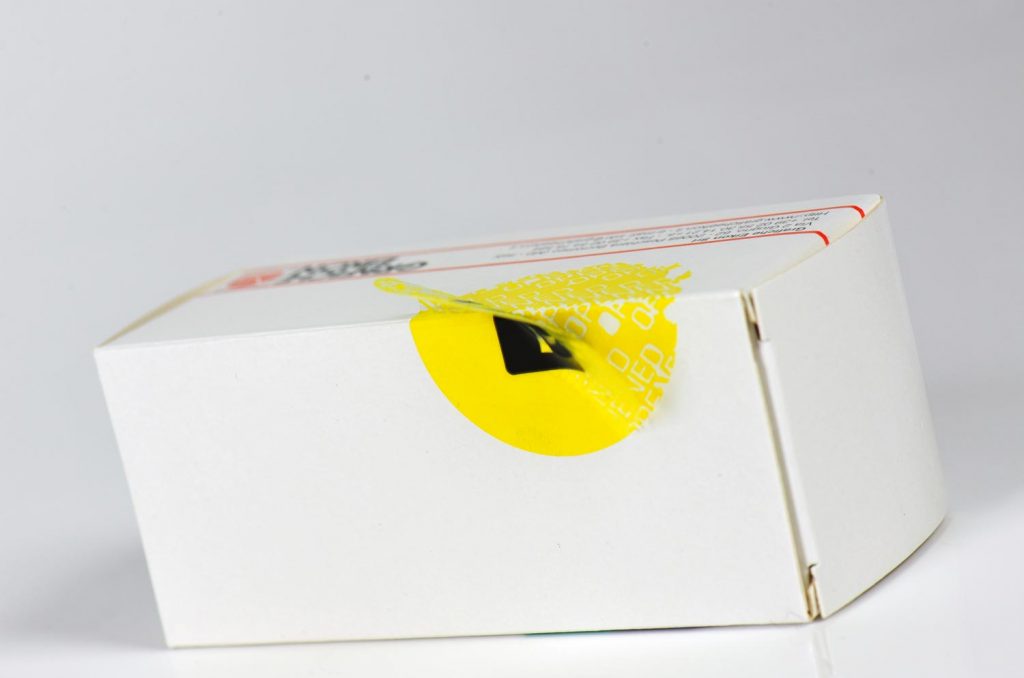
Void Labels
Void labels have been one of the first highly effective answers to the problem of tampering in pharmaceutical industry. These seals are usually very visible because they are characterized by a coloured pigmentation. If removed, they partially leave their colour on the product, marking the protected product with the standard writing “Void” or any other custom-made word.
Even if such writings on the product should be removed, when the label is repositioned it would show the same warning in negative.
Void labels can be made of different plastic materials and in custom-made thicknesses according to specific needs.
Tamper Evident solutions: Arca's offer
In compliance with Directive 2011-62-CE, Arca Etichette’s offer is structured with a range of solutions for pharmaceutical serialization and for the anti-tamper of pharmaceutical boxes and containers.
For this requirement, Arca can offer two highly effective tamper-evident solutions: Wipe-Out & Void seals/labels.
Even larger surface areas, such as boxes containing products or other bulky packaging, can be protected using Wipe-Out and Void type tamper-proof security tapes.
The wide range of materials for security label production and printing allows a safe use both on flat surfaces and on corner applications.
Our tamper-evident labels can also be combined with special security inks, that can function as anti-counterfeiting and security elements themselves.
Wipe-out seals
A real innovation, patented by Arca Etichette and designed to effectively cope with the problem of tampering, these labels can seal any type of box and container, making evident all attempts at illegal break-ins, thus discouraging product and content theft and sophistication.
The particular combination of materials and the specific types of adhesives used make it virtually impossible to detach the label from the container without breaking it into pieces, avoiding its repositioning after the break-in.
Wipe-Out labels comprise two layers:
- A lower layer made of a fragile destructible material
- An upper layer made of a transparent plastic material
If any attempt is made to remove it, the label will effectively be destroyed by three joint factors:
- the plastic layer tends to separate from the lower paper layer, stripping away the print;
- the lower layer tends to self-destruct, due to its inherent fragility;
- the destruction of the lower layer is ensured by the special pattern of pre-cuts over the entire label, extending to its perimeter.
For applications on medicine containers, Arca Etichette offers a range of materials and adhesives specifically designed for the pharmaceutical industry.
Void Labels
Void labels have been one of the first highly effective answers to the problem of tampering in pharmaceutical industry. These seals are usually very visible because they are characterized by a coloured pigmentation. If removed, they partially leave their colour on the product, marking the protected product with the standard writing “Void” or any other custom-made word.
Even if such writings on the product should be removed, when the label is repositioned it would show the same warning in negative.
Void labels can be made of different plastic materials and in custom-made thicknesses according to specific needs.
Label application on pharmaceutical containers
In addition to the creation and printing of effective security and anti-tampering seals, Arca Etichette’s offer is completed with high-tech machines and systems for the application of these solutions. The Systems Division of Arca designs and manufactures machines with all the features that make them suitable for working in the pharmaceutical market. All our processes strictly comply to GAMP guidelines (Good Automated Manufacturing Practice).
Label application on pharmaceutical containers
In addition to the creation and printing of effective security and anti-tampering seals, Arca Etichette’s offer is completed with high-tech machines and systems for the application of these solutions. The Systems Division of Arca designs and manufactures machines with all the features that make them suitable for working in the pharmaceutical market. All our processes strictly comply to GAMP guidelines (Good Automated Manufacturing Practice).